The APF Drug Database is a critical resource for patients with AHP or Acute Porphyrias (AIP, VP, HCP and ADP). It plays a vital role in ensuring the safety and well-being of individuals living with this rare disease. This information is provided as a resource to health care providers to help reduce the risk of unnecessary suffering, hospitalizations, and medical complications for patients.
This database is based on a compilation of expert assessments of the potential of drugs to trigger attacks or worsen the symptoms of AHP, or Acute Porphyrias (AIP, VP, HCP & ADP), based on the available medical evidence. However, this evidence is not always complete, and some medications affect individual patients differently, which may lead to some degree of uncertainty.
The information provided in the Drug Database pertains only to the risk of an attack of AHP, or Acute Porphyrias, associated with medication. All safety issues and the appropriate use of any drug should be reviewed with a medical provider before taking medication.
The information in the database is meant for general informational purposes to health care professionals and is subject to change. The prescription of drugs to a patient with acute porphyria is solely a matter between a patient and their physician
Disclaimer, Terms of Use, and Copyright Notice for the Drug Database for Acute Porphyria
By using this website and accessing the Drug Database, you expressly accept and agree to the following terms and conditions:
Disclaimer and Terms and Conditions of Use:
The Drug Database for Acute Porphyria is property of the American Porphyria Foundation (APF). The information provided in this database is intended solely for general informational purposes for use by healthcare professionals. While it may be of interest to patients, any and all decisions regarding use or non-use of any medication in acute porphyria are ultimately the responsibility of the patient and their health care provider. APF does not provide medical advice and the information contained herein should not be considered medical advice.
The database comprises expert assessments of the potential of drugs to provoke porphyria attacks in patients with acute hepatic porphyria, including Acute Intermittent Porphyria (AIP), Variegate Porphyria (VP), Hereditary Coproporphyria (HCP), and ALA Dehydratase Deficiency Porphyria (ADP). It offers guidance for HCPs based on a thorough evaluation of international clinical experience, published case reports, and theoretical considerations. The information presented in this database by APF is presented in good faith for general informational purposes solely based on the foregoing and is not intended to represent the opinion of the APF nor is it intended to be a factual or medical conclusion. APF does not make any warranties or guarantees about the completeness, reliability, and accuracy of the information. Users acknowledge that no action should be taken based on the information contained herein and any action or inaction taken based on the information found on this website is strictly at ones own risk.
APF accepts no responsibility for any errors, differences of opinion, or adverse consequences arising from or relating to the use of information published on this website APF will not be liable for any losses and/or damages in connection with the use of the Drug Database and, as a condition of using or accessing the Drug Database, you hereby agree to hold the APF, its employees, consultants and agents harmless from any and all claims, damages and liability based on your use of this database and the information contained herein to the maximum extent permitted by applicable laws.
New information may be periodically added to the database, and existing data may be subject to change. While efforts are made to keep the database up to date and operational, APF takes no responsibility for and will not be liable for any errors or temporary unavailability due to technical issues regardless of cause.
Limitations and restrictions on the use of data from this website are outlined in the Copyright section. If using data from this website, proper referencing is required. Refer to:
The Drug Database for Acute Porphyria. APF.
By using this website and/or the database, users expressly consent to the disclaimer and agree to its terms of use.
Copyright Notice:
This website and its content are the copyright of The American Porphyria Foundation (APF). All rights reserved.
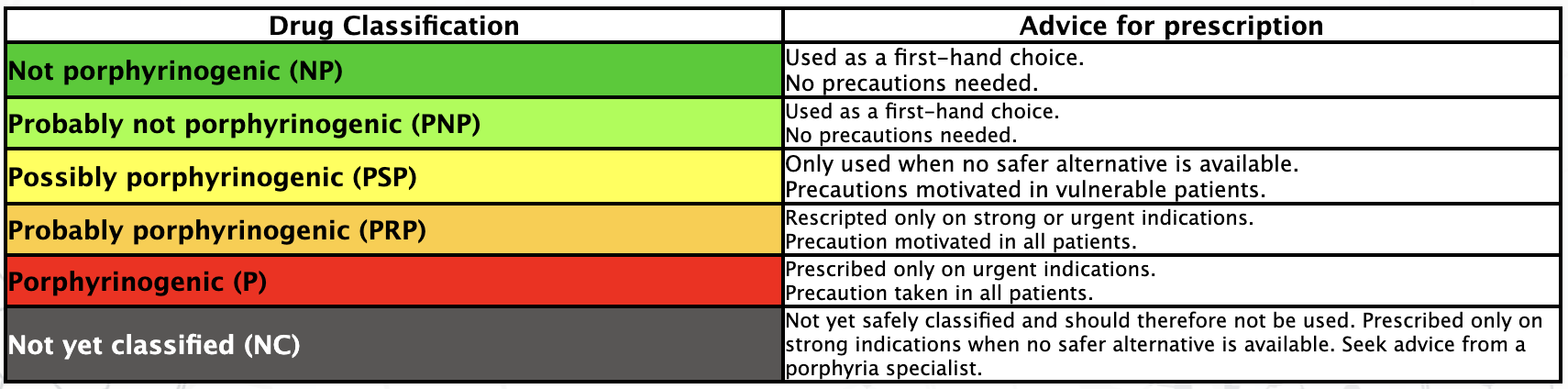
The information in this drug database for acute Porphyria is meant to be used by health care professionals, even though it may also be of interest to patients. Medication use in acute Porphyria should always be discussed jointly by patient and health care professional, and if in doubt, further advice should be obtained.
The database contains expert assessments of the potential of drugs to provoke attacks of acute Porphyria. The database provides guidance based on a very careful evaluation of international clinical experience, published case reports, previously published drug lists, and theoretical considerations. The quality of patient reports used in the assessments is however often unsatisfactory and for most assessments the available clinical evidence is scarce. In addition, the theoretical models for the mechanisms of action of drugs in the Porphyria disease are still incomplete.
The information in this database, which contains some degree of uncertainty, is meant as guidance to health care professionals. It must be made clear that the prescription of drugs to a patient with acute Porphyria is entirely at the risk of the physician in charge.
The American Porphyria Foundation and the staff accept no responsibility for any error, difference of opinion or any adverse consequences arising from or occasioned by the use of information published on this website.
**Preferred Browsers are Google Chrome, Firefox, Internet Explorer, Opera, and Safari.**
The following individuals, who contributed their knowledge and expertise to the development of this drug list, receive our sincere thanks: