
Please note: The following content was not reviewed by Alnylam Pharmaceuticals.
GIVLAARI is a treatment used to reduce acute hepatic porphyria (AHP) attacks in adults.There are 4 types of AHP: acute intermittent porphyria (AIP), variegate porphyria (VP), hereditary coproporphyria (HCP), and ALA-dehydratase deficient porphyria (ADP). GIVLAARI is given once a month as a subcutaneous injection (under the skin) by a healthcare professional.
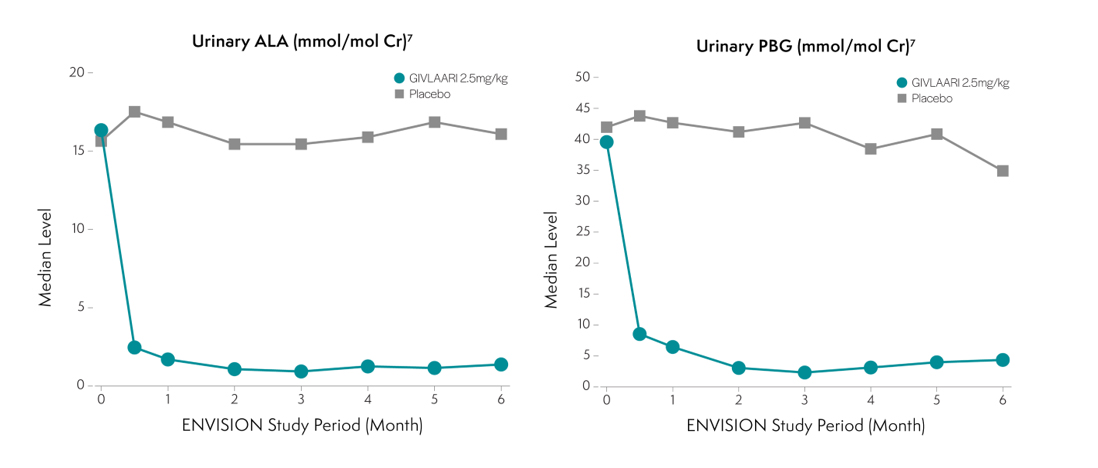
GIVLAARI is a double-stranded small interfering RNA (siRNA) therapeutic specifically targeting ALAS1 mRNA, reducing ALAS1 mRNA levels and leading to reductions in urinary ALA and PBG.1
ALA, delta-aminolevulinic acid; ALAS1, delta-aminolevulinic acid synthase 1; mRNA, messenger RNA; PBG, porphobilinogen.
Disease triggers, such as infections or certain medications, can induce ALAS1 and led to overproduction of the neurotoxic intermediates ALA and PBG 2-3. ALA and PBG accumulate in the liver, and are further released into circulation, thereby causing neurototoxic effects3-5. Neurotoxic effects lead to acute attacks 3-5.
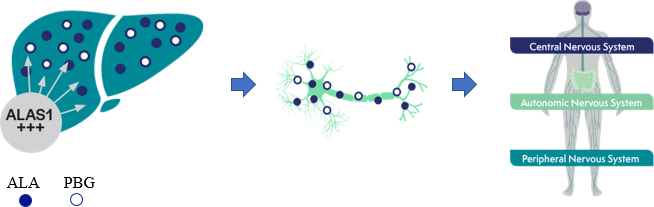
Specifically targeting ALAS1 mRNA for degradation reduces the production of the neurotoxic intermediates ALA and PBG1 . Less ALA and PBG are released into circulation4,6. Reductions of ALA and PBG have been associated with fewer attacks6.
References: 1. GIVLAARI [package insert]. Cambridge, MA: Alnylam Pharmaceuticals, Inc; 2019. 2. Balwani M et al; the Porphyrias Consortium of the Rare Diseases Clinical Research Network. Acute hepatic porphyrias: recommendations for evaluation and long-term management. Hepatology. 2017;66(4):1314-1322. 3. Puy H et al. Porphyrias. Lancet. 2010;375(9718):924-937. 4. Bissell DM et al. Porphyria. N Engl J Med. 2017;377(9):862-872. 5. Bonkovsky HL et al. Pathogenesis and clinical features of the acute hepatic porphyrias (AHPs). Mol Genet Metab. 2019. doi: 10.1016/j.ymgme.2019.03.002. 6. Sardh E et al. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med. 2019;380(6):549-558. 7. Data on file. Alnylam Pharmaceuticals, Inc.
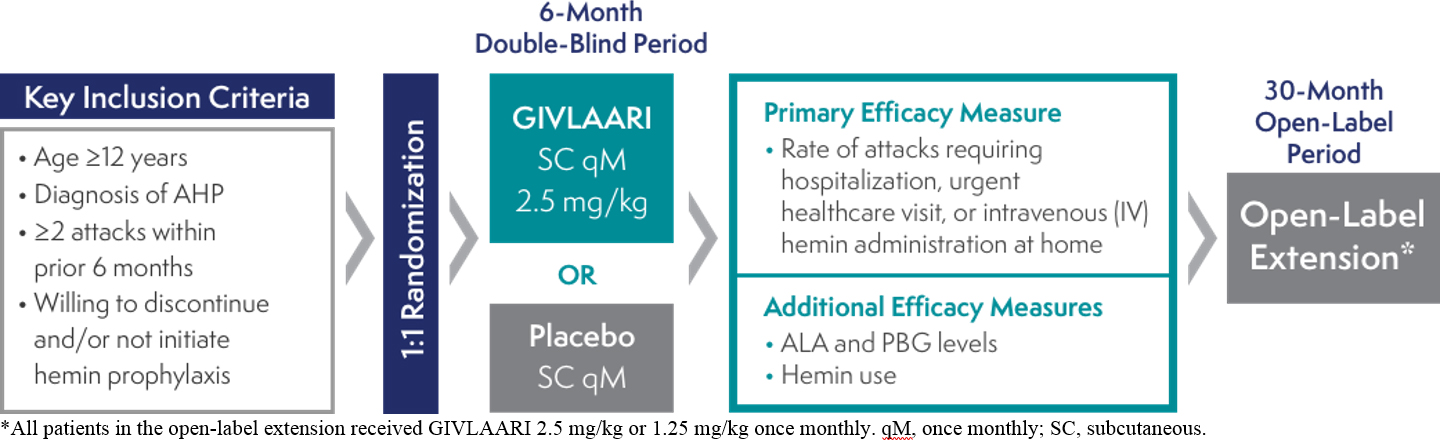
The efficacy and safety of GIVLAARI® (givosiran) were evaluated in ENVISION, a randomized, double-blind, placebo-controlled, multinational Phase 3 study in adult patients with AHP (N=94)1,2
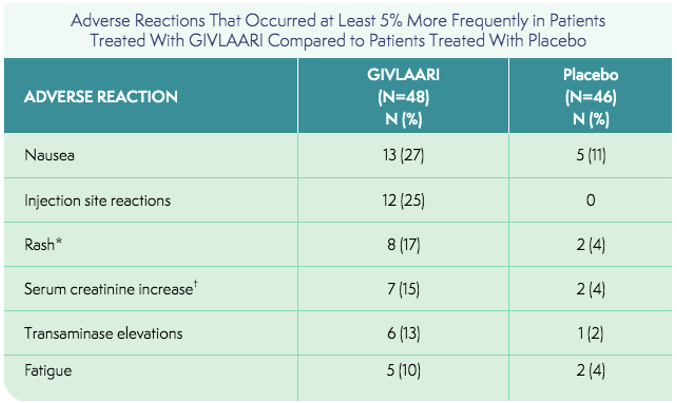
*Grouped term includes pruritus, eczema, erythema, rash, rash pruritic, urticaria.
†Grouped term includes blood creatinine increased, glomerular filtration rate decreased, chronic kidney disease (decreased estimated glomerular filtration rate).
References: 1. GIVLAARI [package insert]. Cambridge, MA: Alnylam Pharmaceuticals, Inc; 2019. 2. Gouya L et al; ENVISION investigators. ENVISION, a Phase 3 study to evaluate the efficacy and safety of givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. Oral presentation at: International Congress on Porphyrins and Porphyrias; September 10, 2019; Milan, Italy. 3. Data on file. Alnylam Pharmaceuticals, Inc. 4. Balwani et al; ENVISION investigators. ENVISION, a Phase 3 study to evaluate the efficacy and safety of givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. Poster presented at: European Association for the Study of the Liver International Liver Congress; April 13, 2019; Vienna, Austria. 5. Sardh E et al. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med. 2019;380(6):549-558.
The recommended dose of GIVLAARI is 2.5 mg/kg administered by a healthcare professional via subcutaneous injection once monthly. Dosing is based on actual body weight. GIVLAARI is intended for subcutaneous use by a healthcare professional only
Reference: 1. GIVLAARI [package insert]. Cambridge, MA: Alnylam Pharmaceuticals, Inc; 2019.